Futures Surge On Gilead Headlines About Remdesivir
Gilead Shares are halted on Wednesday morning as the drugmaker releases positive-sounding headlines about its potential coronavirus drug remdesivir.
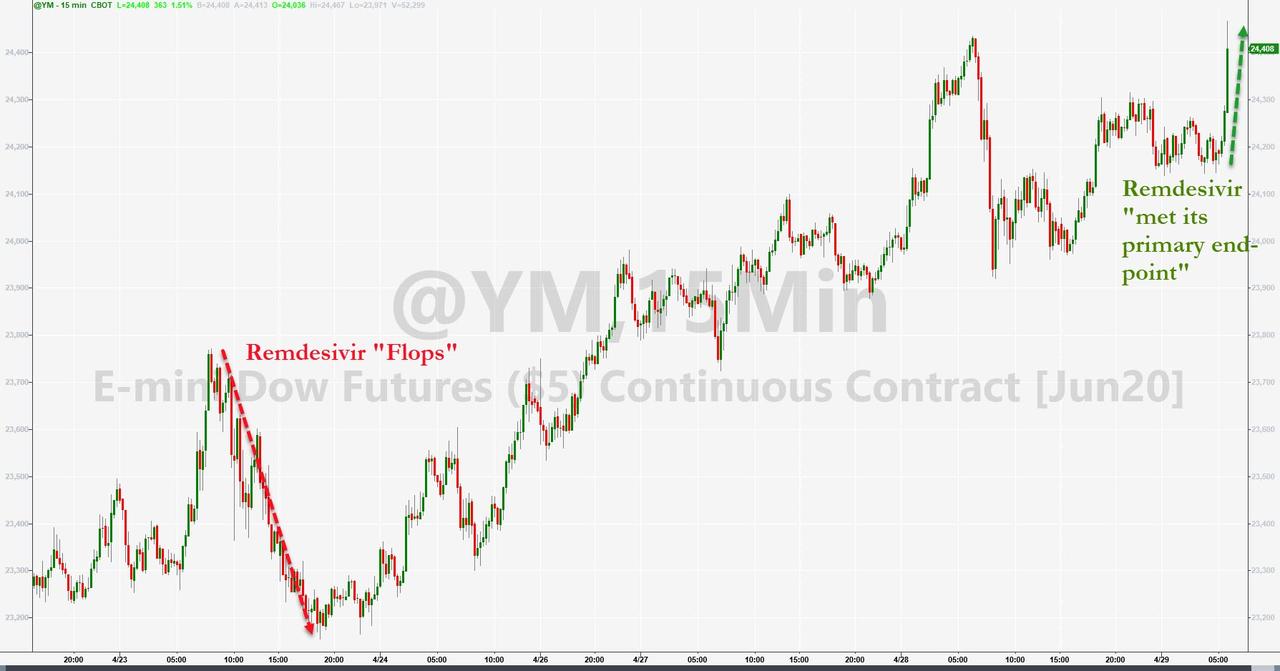
An initial report noted that Gilead is aware of positive data emerging from the National Institute of Allergy and Infectious Diseases’ (NIAID) study of the investigational antiviral remdesivir for the treatment of COVID-19.
We understand that the trial has met its primary endpoint and that NIAID will provide detailed information at an upcoming briefing. Remdesivir is not yet licensed or approved anywhere globally and has not yet been demonstrated to be safe or effective for the treatment of COVID-19. Gilead will share additional remdesivir data from the company’s open-label Phase 3 SIMPLE trial in patients with severe COVID-19 disease shortly.
This study will provide information on whether a shorter, 5-day duration of therapy may have similar efficacy and safety as the 10-day treatment course evaluated in the NIAID trial and other ongoing trials.
Gilead expects data at the end of May from the second SIMPLE study evaluating the 5- and 10-day dosing durations of remdesivir in patients with moderate COVID-19 disease. Gilead will continue to discuss with regulatory authorities the growing data set regarding remdesivir as a potential treatment for COVID-19.
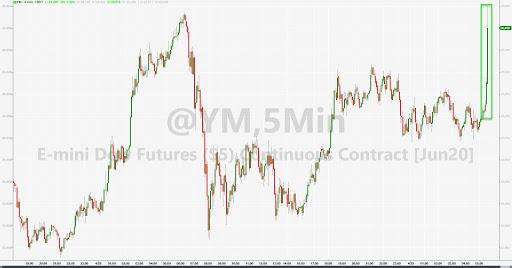
Futures soared on the Gilead report…
After they plunged last week on leaked China data from the WHO suggested the drug was a “flop”…
Additionally, as Bloomberg notes, Gilead issued a statement on its own ‘SIMPLE’ trials, noting positively no new safety signals were identified with remdesivir across either treatment group.
“Unlike traditional drug development, we are attempting to evaluate an investigational agent alongside an evolving global pandemic. Multiple concurrent studies are helping inform whether remdesivir is a safe and effective treatment for COVID-19 and how to best utilize the drug,” said Merdad Parsey, MD, PhD, Chief Medical Officer, Gilead Sciences.
“These study results complement data from the placebo-controlled study of remdesivir conducted by the National Institute for Allergy and Infectious Diseases and help to determine the optimal duration of treatment with remdesivir. The study demonstrates the potential for some patients to be treated with a 5-day regimen, which could significantly expand the number of patients who could be treated with our current supply of remdesivir. This is particularly important in the setting of a pandemic, to help hospitals and healthcare workers treat more patients in urgent need of care.”
Gilead plans to submit the full data for publication in a peer-reviewed journal in the coming weeks, but there are reports that Dr. Anthony Fauci will hold a press conference later today discussing the NIAD/Gilead results suggesting an accelerated approval process, according to former FDA head Dr. Scott Gottlieb.
Remdesivir is not yet licensed or approved anywhere globally and has not yet been demonstrated to be safe or effective for the treatment of COVID-19, but this is an incremental step towards that, although Gottlieb warned this is “not a silver bullet.”
Tyler Durden
Wed, 04/29/2020 – 08:34