The Remdesivir Study Is Finally Out: Drug Only Helped Those On Oxygen, Finds Mortality Too High For Standalone Treatment
Tyler Durden
Fri, 05/22/2020 – 20:45
Remember when the market soared on several days in April on the Facui-touted Remdesivir study which, according to StatNews and various other unofficial sources of rumors, was a smashing success only for the optimism to fizzle as many questions emerged, and as the Gilead drug quietly faded from the public’s consciousness and was replaced by various coronavirus vaccine candidates such as those made by the greatly hyped Moderna (whose insiders just can’t stop selling company stock).
Meanwhile, those who were waiting for the official version of Remdesivir’s effectiveness had to do so until 6pm on a Friday before a long holiday, and for good reason…
Friday 6 pm. Fking ridiculous. https://t.co/6Ze38BfVgN
— Adam Feuerstein (@adamfeuerstein) May 22, 2020
… According to a pivotal study published in the New England Journal of Medicine late on Friday, Remdesivir, which was authorized to treat Covid-19 in a group of 1063 adults and children (split into two groups, one receiving placebo instead of remdesivir) who need i) supplemental oxygen, ii) a ventilator or iii) extracorporeal membrane oxygenation (ECMO), only significantly helped those on supplemental oxygen.
Meanwhile, and explaining the 6pm release on a Friday, the study also found no marked benefit from remdesivir for those who were healthier and didn’t need oxygen or those who were sicker, requiring a ventilator or a heart-lung bypass machine.
The NEJM, almost apologetically, stated that “the lack of benefit seen in the other groups might have stemmed from a smaller number of patients in each group.”
Still, as a result of the partial benefit for patients in the supplemental oxygen group, the study from the National Institute of Allergy and Infectious Diseases was evaluated early and led to the authorization of remdesivir before the full trial was completed.
Our findings highlight the need to identify Covid-19 cases and start antiviral treatment before the pulmonary disease progresses to require mechanical ventilation.
Some more details on the study, which was a “rank test of the time to recovery with remdesivir as compared with placebo, with stratification by disease severity”:
The primary outcome measure was the time to recovery, defined as the first day, during the 28 days after enrollment, on which a patient satisfied categories 1, 2, or 3 on the eight-category ordinal scale. The categories are as follows:
- not hospitalized, no limitations of activities;
- not hospitalized, limitation of activities, home oxygen requirement, or both;
- hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care (used if hospitalization was extended for infection-control reasons);
- hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (Covid-19–related or other medical conditions);
- 5, hospitalized, requiring any supplemental oxygen;
- hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices;
- hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and
- death.
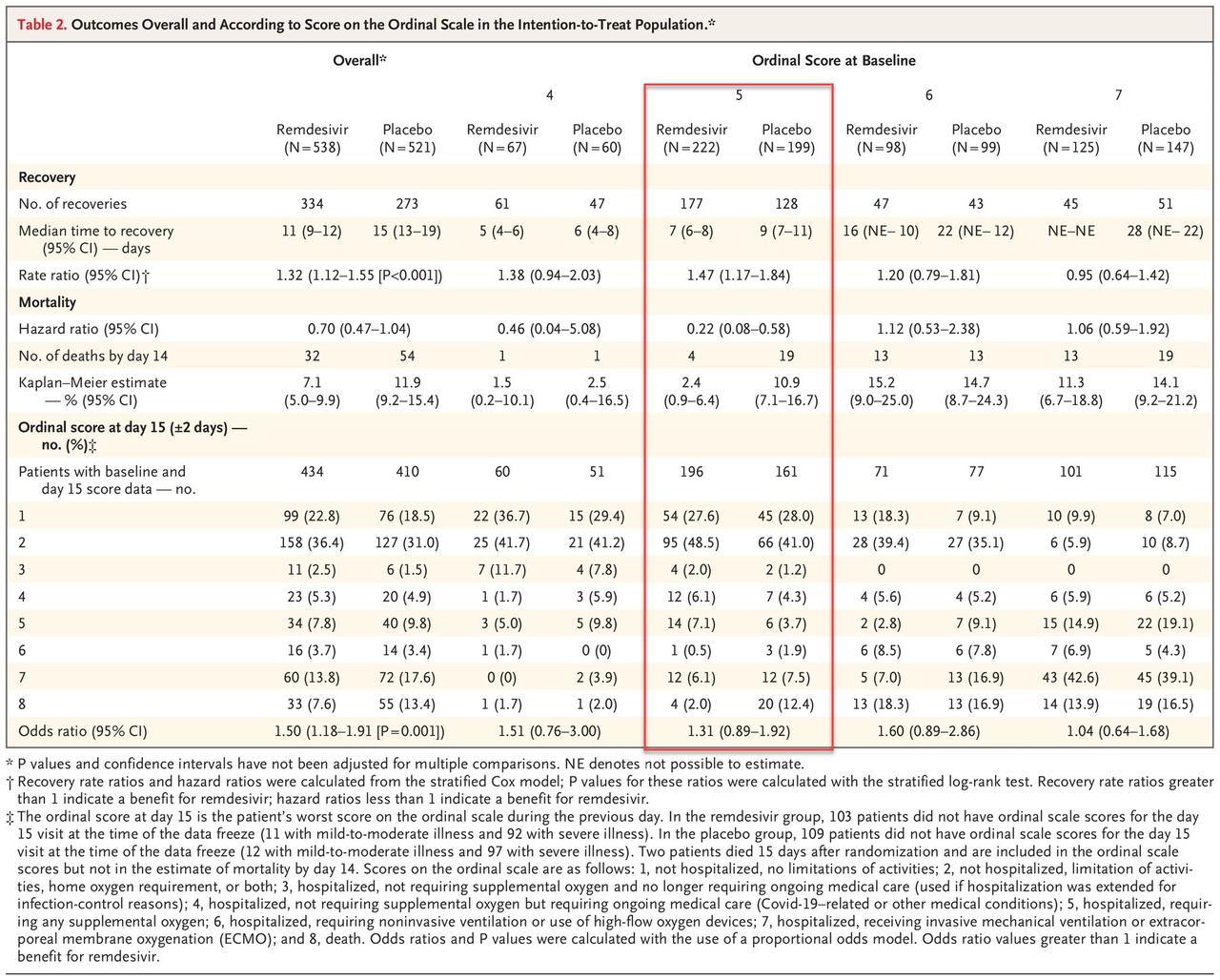
The results are summarized below, highlighting the only group that showed a statistically significant improvement in outcomes as a result of taking the drug vs placebo.
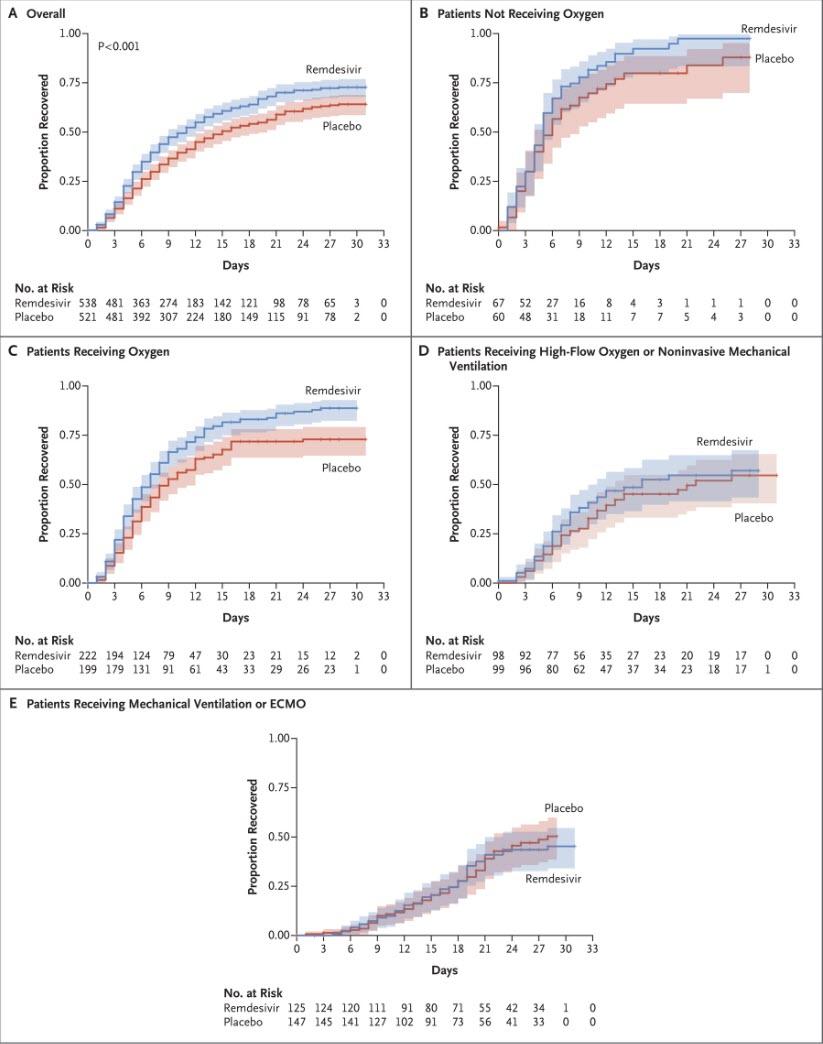
A visual representation of the outcomes is below; it shows that whereas there was a modest benefit only to patients who were receiving oxygen, the results were statistically insignificant vs placebo for patients not receiving oxygen, while in a surprising twist patients on high-flow oxygen or mechanical ventilator/ECMO did modestly better in the placebo group than those taking remdesivir. Also, the overall results showed a very modest, but not statistically significant improvement in the remdesivir group vs placebo (box A).
Another disappointment: the study found that overall “mortality was numerically lower in the remdesivir group than in the placebo group, but the difference was not significant“, in other words the alleged “miracle drug” has largely the same effect as a placebo in terms of overall disease mortality.
The study authors also note that the “findings in our trial should be compared with those observed in a randomized trial from China in which 237 patients were enrolled (158 assigned to remdesivir and 79 to placebo)…. That trial failed to complete full enrollment (owing to the end of the outbreak), had lower power than the present trial (owing to the smaller sample size and a 2:1 randomization), and was unable to demonstrate any statistically significant clinical benefits of remdesivir.“
Finally, the study found that while mortality was modestly lower for the remdesivir arm, it was not significantly so, at 7.1% at 14 days on drug versus 11.9% on placebo.
In conclusion, while the “preliminary findings support the use of remdesivir for patients who are hospitalized with Covid-19 and require supplemental oxygen therapy” the study goes on to warn that “given high mortality despite the use of remdesivir, it is clear that treatment with an antiviral drug alone is not likely to be sufficient.”
The study’s recommendation:
Future strategies should evaluate antiviral agents in combination with other therapeutic approaches or combinations of antiviral agents to continue to improve patient outcomes in Covid-19.
So a generally disappointing outcome, one which would lead to a drop in the market. Nonsense: think of all the spin, and why this is in fact great news for stocks: Remdesivir may be a dud as a “silver bullet” to curing covid, leading to statistically significant improvement in only a very limited subset of infected patients and “high mortality” for those taking it, but at least the algos will have a whole lot of other “miracle drugs” to levitate them as optimism that the next remdesivir is just around the corner. In short: rinse, rumor, and repeat… and then save the bad news for 6pm on a Friday.
Oh, and for those asking about the “official” reason why the NE Journal of Medicine waited until just the right time to make sure nobody reads the results, here it is:
I asked NEJM spox to explain the Friday 6 pm release of the remdesivir study. Her response is below. pic.twitter.com/WjNGyUv7sH
— Adam Feuerstein (@adamfeuerstein) May 22, 2020
The full study is available here.