Oxford-AstraZeneca COVID-19 Vaccine Trials Show “No Adverse Effects”
Tyler Durden
Mon, 07/20/2020 – 09:36
Update (0935ET): A coronavirus vaccine candidate developed by Oxford and AstraZeneca has shown promise in an early trial which found it to be safe for human consumption while reliably producing antibodies that are effective at stopping the virus.
In what looked like a coordinated one-two punch, one of the top researchers leading the Oxford-Astrazeneca trials said in an interview published Monday morning that the research was making “good progress”. Minutes later, the Lancet published the first Phase 1/2 trial results, which showed that the Oxford-AstraZeneca vaccine caused “robust immune responses” and was “tolerated” by all study subjects.
That interview was published Monday morning in the US, just minutes before the Lancet released the results of a Phase 1/2 study of the Oxford-AZ vaccine, the most highly anticipated COVID-19 news of the day.
There are currently more than 137 vaccine candidates undergoing preclinical development, and 23 in early clinical development, according to WHO. Of these, candidates from Moderna and the Oxford-AstraZeneca partnership are two of the most closely followed prototypes. Governments have already started ordering the vaccine from Moderna, even though approval is still months, perhaps years, away.
According to the Lancet, research has shown that vaccine candidates from Cansino and Astra-Oxford trial have been making good progress, and while they couldn’t say much conclusively, the Astra-Oxford trial showed no worrisome “adverse effects”.
The Phase 1/2 trial, one of the first human studies of the vaccine, showed an appropriate “immune response”. Patients who received 2 doses instead of one saw a stronger response. All patients who received the vaccine generated the desired immune response.
Oxford’s candidate “showed an acceptable safety profile, and homologous boosting increased antibody responses. These results “support large scale evaluation of this candidate vaccine in an ongoing phase 3 program.” The Oxford-AZ study included 1,077 participants spread across 5 test sites in and around the UK.
By comparison, Moderna has released press releases touting findings from studies with fewer than 100 patients. The fact that 8 patients developed neutralizing antibodies in a study that involved dozens of additional subjects was apparently news enough for Moderna, which released a market-pumping press release on those findings a few weeks back.
Though to be sure, not everybody was impressed.
$AZN VACCINE DATA LOOKS ‘MEDIOCRE’ – STAT’S FEUERSTEIN
— *Walter Bloomberg (@DeItaOne) July 20, 2020
In the study, researchers measured the number of antibodies, and the strength of the immune response, after administering single doses and double doses of the vaccine to various groups of study subjects, and compared those results with a control group who received another vaccine. Pain and swelling caused by the injection were easily treated with paracetemol.
There were no serious adverse events related to ChAdOx1 nCoV-19. In the ChAdOx1 nCoV-19 group, spike-specific T-cell responses peaked on day 14 (median 856 spot-forming cells per million peripheral blood mononuclear cells, IQR 493–1802; n=43). Anti-spike IgG responses rose by day 28 (median 157 ELISA units [EU], 96–317; n=127), and were boosted following a second dose (639 EU, 360–792; n=10). Neutralising antibody responses against SARS-CoV-2 were detected in 32 (91%) of 35 participants after a single dose when measured in MNA80 and in 35 (100%) participants when measured in PRNT50. After a booster dose, all participants had neutralising activity (nine of nine in MNA80 at day 42 and ten of ten in Marburg VN on day 56). Neutralising antibody responses correlated strongly with antibody levels measured by ELISA (R²=0·67 by Marburg VN; p<0·001).
The result: The vaccine candidate has been deemed safe enough to move on to ‘Phase 3’, which would involve large-scale human trials.
ChAdOx1 nCoV-19 showed an acceptable safety profile, and homologous boosting increased antibody responses. These results, together with the induction of both humoral and cellular immune responses, support largescale evaluation of this candidate vaccine in an ongoing phase 3 programme.
Read the full Lancet paper below:
s 0140673620316044 by Zerohedge on Scribd
* * *
Researchers working on the AstraZeneca-Oxford vaccine have reportedly told the press that the ‘Phase 1/2’ trials have made “good” progress.
“We are seeing very good immune responses, not just on neutralizing antibodies but of T-cells as well,” said Adrian Hill, head of Oxford’s Jenner Institute, in an interview. “We’re stimulating both arms of the immune system.”
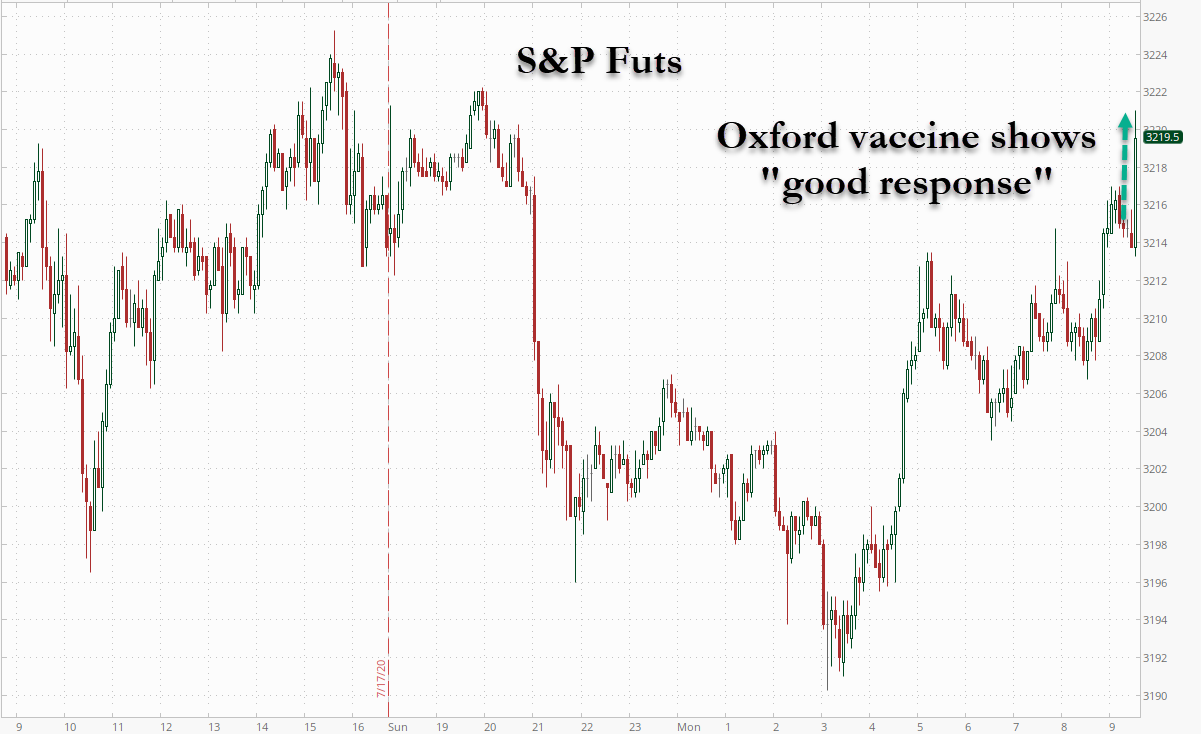
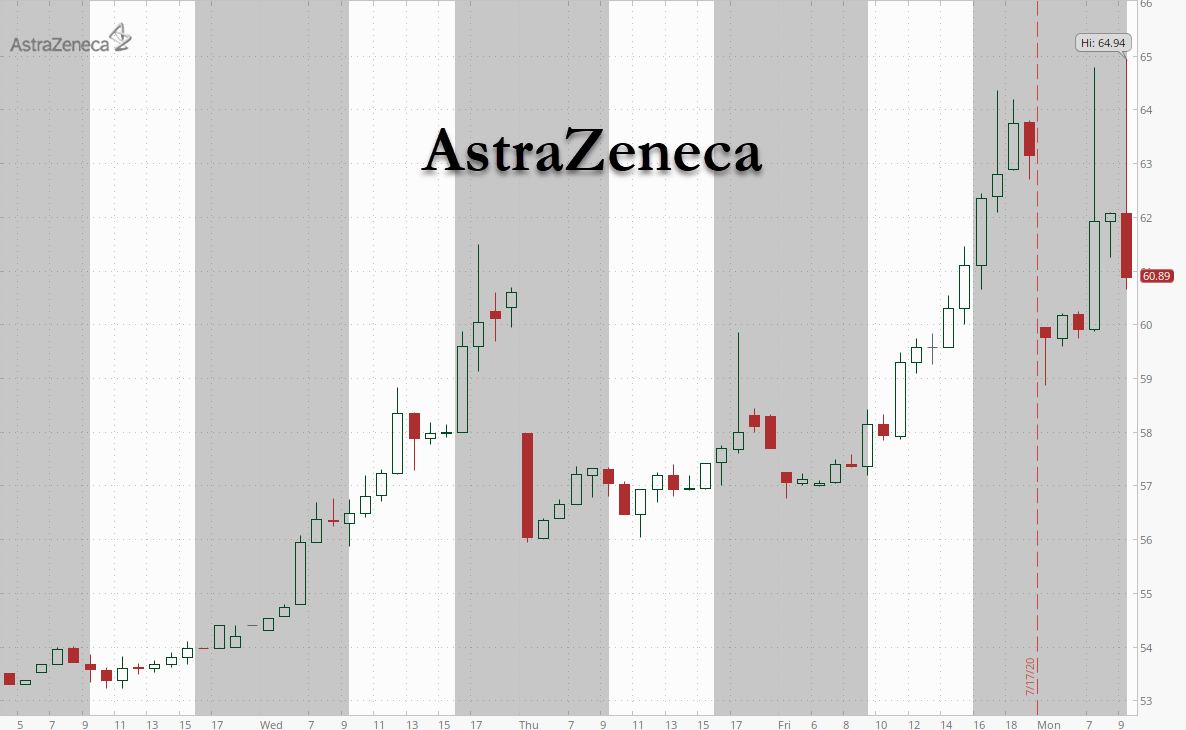
The news sent AstraZeneca shares surging higher, along with the broader market, as investors await the release of more results from early stage vaccine trials.
AstraZeneca shares surged 9% on the news before fading into the red.