Multiple States And FDA Warn Of “Careless” Rapid Antigen Testing In Asymptomatic COVID Cases
Tyler Durden
Mon, 11/09/2020 – 20:20
As if there wasn’t enough confusion and broad misunderstanding going around about Covid, the states of Louisiana and Oregon are now warning against using rapid, low cost antigen tests in asymptomatic people to try and determine whether or not they are positive with coronavirus. Oh, and so is the Food and Drug Administration…
The appeal of these tests is that they can be spread widely and cheaply, giving the illusion of control over the virus to individuals and organizations that use them for quick blanket testing.
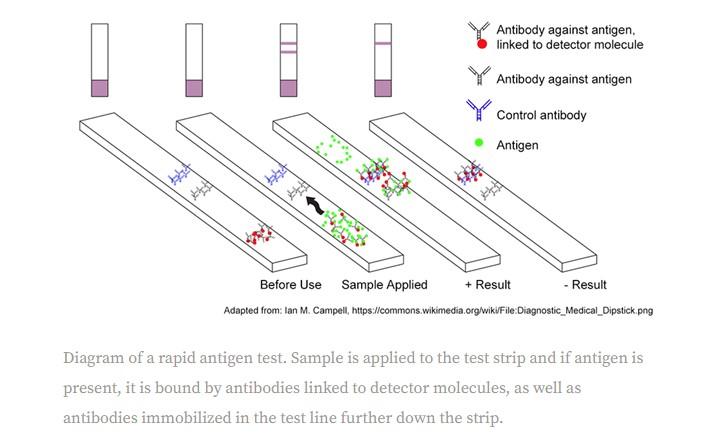
But these tests could “miss some infections that can be picked up by costlier gold-standard assays, and can incorrectly return positive results,” according to Bloomberg.
As a result, the state is not recommending the tests to people without symptoms, who have not been exposed to someone with a positive test. The Louisiana Department of Health also says that people who undergo antigen tests should be made aware of its limitations.
Joseph Kanter, interim assistant secretary for the state’s Office of Public Health, said: “On the one hand, we have technology and testing platforms like this one which are new and likely valuable. And everybody has an interest in getting them to people that could benefit from them as quick as possible. On the other hand, we don’t have great data on them yet.”
The Oregon Health Authority has also warned against using the tests in asymptomatic people without confirmed Covid-19 exposure.
Mark McClellan, director of the Duke-Margolis Center for Health Policy at Duke University, commented that antigen tests for one time use risks the chance of missing infections: “For people looking to one-time use of Covid antigen tests as a way to go back to normal, we’re not in that situation now. This is one more layer of protection as we try to reopen and get through the rest of the pandemic.”
On Tuesday, the FDA also warned about the tests, saying they can produce “incorrect positive results”. Nursing homes and other care settings have reported false positives from antigen tests, the FDA noted.
These warnings apply to tests like Abbot’s $5 BinaxNOW test that the U.S. government is spending $750 million on. Now, state health officials are reportedly “increasingly concerned that people without symptoms should be screened with more costly but more reliable polymerase chain reaction assays”.
Jeff Engel, a senior adviser at the Council of State and Territorial Epidemiologists, said: “HHS made this purchase without any studies on the novel use in which they’re deploying these tests. I think that’s careless.”
It’s also one of the infinite reminders that government is not only an abhorrent allocator of other peoples’ capital, but is also flailing wildly in trying to control a virus that is casually making its way across the globe regardless of what preventative measures we take.
HHS official Brett Giroir responded: “We do support asymptomatic testing being used. That is the only way that you’re going to screen millions of people a month.”
Abbot responded: “Widespread, affordable rapid antigen testing helps slow the virus’s spread, and tests like BinaxNOW, when used as intended, can detect those most likely to be infectious.”