There is a major scandal brewing at The Food and Drug Administration (FDA) after the government refused to name the company responsible for manufacturing more than two dozen eye drop products that could cause several health problems, including total blindness.
The Gateway Pundit previously reported that the FDA issued a warning to customers NOT to purchase the 27 products being sold at some of the nation’s most prominent retailers. The impacted products were marketed under the brands CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up&Up, WalMart, and Velocity Pharma.
As a reminder, this was the full list of potentially contaminated products:

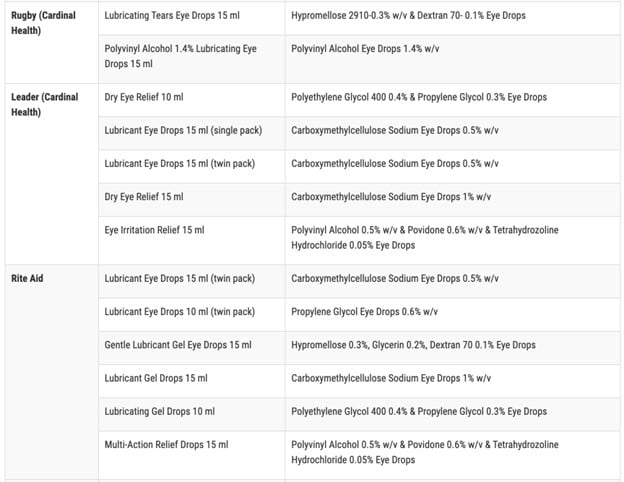
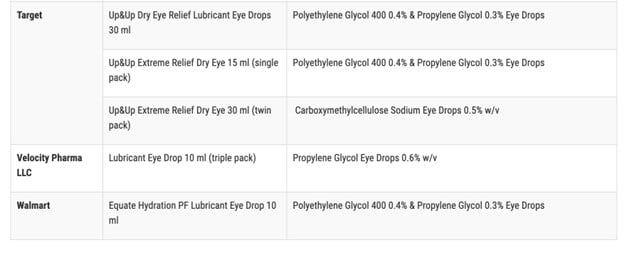
At the time, TGP wondered what the FDA was hiding from the public when they would not identify the manufacturer responsible for these dangerous eye care products. They did not even bother mentioning what country the manufacturer was located in.
On Wednesday, the FDA FINALLY decided name the manufacturer and allowed it to initiate a VOLUNTARY recall of all their products from stores the same day. The company is called Kilitch Healthcare India Limited, which is located in Mumbai, India.
As Gateway Pundit readers know, a different Indian eye drop manufacturer named Global Pharma Healthcare earlier this year was responsible for a deadly outbreak that killed three people with several more losing their vision. Now we have ANOTHER Indian company that potentially endangered American lives while the FDA said nothing.
The news only gets worse and more infuriating. We also learned from the Associated Press that Kilitch Healthcare India Limited has a host of disgusting manufacturing and sanitation problems at their plant in Mumbai including barefoot workers, cracked floors, and altered records. And the FDA was fully aware of this from the start.
These sick and corrupt details emerged after the FDA visited their plant late last month, roughly the same time they first alerted the American public on the eye drop products.
From the Associated Press:
Food and Drug Administration officials uncovered more than a dozen problems at the Mumbai plant operated by Kilitch Healthcare India, according to a preliminary inspection report posted by the agency. The factory produced more than two dozen varieties of eyedrops that were subject to an FDA safety warning last month.
The products were sold by CVS Health, Target, Rite Aid and other national retailers who said they would be removed from store shelves. New details about the plant’s problems emerged after FDA inspectors visited the plant late last month.
Agency inspectors documented factory workers not wearing masks, gloves and gowns and working barefoot in areas that are supposed to be sterile. A manager told FDA officials “that this is their standard practice,” according to the report.
Elsewhere, FDA staff noted cracked floors, plus water stains and peeling paint on walls and ceilings.
As the AP notes, the FDA report suggests these factory officials engaged in fraudulent behavior on a regular basis. For example, they would routinely omit or falsify test results.
One example cited by the AP is the plant would refuse to document a bacterial sample that could trigger “an alert or action limit.” Instead, Kilitch Healthcare India would perform “additional cleaning” and then record an item that indicated “sterility.” This happened two to three times a month.
And guess what the FDA’s answer is planning to do? Issue a mere warning letter to the corrupt Indian company.
The FDA KNEW that this Indian manufacturer was responsible from day one and had full awareness regarding the horrific conditions at the plant. Yet they CHOSE to keep this information hidden from the public for almost three weeks.
Meanwhile, the products were still available at some stores in America during that time.
Then, they let Kilitch Healthcare India issue a VOLUNTARY recall instead of immediately demanding one and alerting congressional authorities to this potential health catastrophe. What were these officials motivations? Were they worried about appearing xenophobic? Did they have a corrupt relationship with this Indian manufacturer?
Now, their answer to combatting this crisis is issuing a warning letter. What a joke.
Congress must schedule hearings to demand these answers from FDA officials and hold them accountable for dropping the ball. If not, people will die just like they did earlier in the year.
The post Foreign Manufacturer Responsible for Eye Drop Products Causing Potential BLINDNESS Finally Revealed – FDA Discovered Several DISGUSTING Conditions at Manufacturer that Were “Standard Practice” and Covered it up for WEEKS! appeared first on The Gateway Pundit.